Last updated on: July 11th, 2019
Pharmacokinetics
- Pharmacokinetics: what human body does to a drug; involves: absorption, distribution, metabolism, excretion.
- Pharmacodynamics: what the drug does to human body.
ADR
- Med guides: required for original prescription and each refill. Not necessary to dispense to inpatients because they are being monitored. E.g: anticonvulsants, antidepressants, long-acting opioids, NSAIDS, ADHD and Strattera.
- Reporting: side effects, adverse events and allergies should be reported to FDA’s MedWatch program (FAERS), vaccines are reported under a different program VAERS.
- Phase IV: post-marking safety surveillance program.
- Mild rash: opioids cause a non-allergic release of histamine from mast cells, causing itches and hives.
- Photosensitivity: diuretics, quinolones, sulfa antibiotics, TCN, retinoids, amiodarone, methotrexate, etc.
- TTP (thrombotic thrombocytopenic purpura): clots throughout the body; clopidogrel, ticlopidine.
- PCN and sulfonamides: two classes that cause the most drug allergies, each pharmacy has separate trays for them.
- Sulfa
- Sulfamethoxazole, sulfasalazine, sulfadiazine, sulfisaxole, sulfonamides (thiazide and loop diuretics), sulfonylurea, acetazolamide, zonisamide, celecoxib.
- Sulfate or sulfite (preservative) do not cross react with sulfonamides.
- Biologics: Common to cause hypersensitivity reactions
- Peanuts and soy
- They belong to the same family and can cross react.
- Drugs: clevidipine (Cleviprex), propofol (Diprivan), progresterone (Prometrium) capsules.
- Eggs: Drugs to avoid: clevidipine (Cleviprex), propofol (Diprivan), yellow fever vaccine.
Absorption
- occurs only if drug is administered extravascularly (non-IV: oral, SL, buccal, IM, transdermal, inhaled, rectal etc.)
Distribution
- a process moves from systemic circulation to tissues and organs.
- Factors: high lipophilicity, low MW, unionized, and low protein binding has greater drug distribution.
- Albumin is the primary protein responsible for protein binding, only the unbound drugs exert therapeutic actions.
- If albumin level is low, Ca and phenytoin levels need to be corrected.
- Ca corrected= reported Ca + [(4-albumin)x0.8]
- Phenytoin corrected (mcg/mL) = total phenytoin measured/ [(0.2xalbumin)+ 0.1]
- Volume of distribution (Vd)
- How large of area the drug distribute to, is based on the properties of drug
- Vd = Dose of drug/ plasma concentration
- Practice: 400mg of tobramycin is administered and the level drawn is 20 mg/L, what’s the volume of distribution?
- Vd = 400mg/20mg/L = 20L
Metabolism
- Parent drug gets converted from its original chemical structure to metabolites to facilitate elimination from body.
- Gut and liver are the primary sites due to higher level of enzyme presence.
- First pass metabolism: drug enters stomach then intestine, after gut, to liver, some drugs go through CYP metabolism before reaching systemic circulation. Lidocaine can not be give PO, because first pass is so extensive and greatly reduce its bioavailability, and can only be given IV.
- Enzyme metabolism
- Phase I (oxidation, reduction, hydrolysis): either inactive the drug or convert drug to its active form.
- Phase II (conjugation): break of carbon bond or add a functional group. E.g: add a -OH group makes a drug more hydrophilic to stay in blood then pass through kidney. Glucuronidation and other phase II reactions create compound more easily excreted through bile and urine.
Excretion
- Clearance (Cl) describes efficacy of drug removal from the body
- Cl = Rate of elimination / Concentration
- Practice: tobramycin is given to a patient and urine sample is collected 4 hours after administration. 300mg drugs were eliminated, and the plasma concentration was 15 mg/L. calculate the patient’s tobramycin clearance
- Cl = (300mg/4) / 15 = 5 L/hr
- AUC (area under curve): most reliable measure of bioavailability (F)
- Cl = F x dose / AUC
- Practice: a patient receives gentamycin IV 500mg QD, AUC 80 mgxhr/L, calculate the patient’s gentamycin clearance
- Cl = 1 x 500 / 80 = 6.25 L/hr
Elimination rate constant (ke)
- Ke = Cl / Vd
- Practice: a drug displays the following characteristics: Vd = 50 L, Cl = 5L/hr, calculate the elimination rate constant.
- Ke = 5L/hr / 50L= 0.1 hr-1
Half-life and steady state
- t1/2: the drug concentration to decrease by 50%, = 0.693/Ke. E.g: it takes 5 hours for theophylline concentration to drop from 16 to 8 mg/L, and another 5 hrs to drop to 4 mg/L.
- steady state: rate of drug intake equals rate of drug elimination; it takes 5 half-lives to reach steady state. (or to remove 95% of drug from the body)
- Practice: TCN has a Vd of 105L and a clearance of 7 L/hr. calculate the half-life and the time required to remove 95% of drug.
- Ke = Cl/Vd = 7/105 = 0.067 hr-1
- T1/2 = 0.693/ke = 0.693 / 0.067 = 10.3 hr
- 3 x 5 = 51.5 hrs
Loading dose
- LD = desired concentration x Vd/ F
Zero & first order kinetics
- Most drugs follow 1st order kinetic where constant percent of drug is removed per time. Double the dose doubles serum concentration.
- Zero order: constant amount of drug (mg) is removed per time. Doubling the dose can more than double the serum concentration.
Michaelis-Menten kinetics
- Phenytoin, theophylline, voriconazole
- Also called saturable, non-linear or mixed-order kinetics.
- Michaelis-Menten constant (Km): the metabolism rate at half of Vmax (maximum rate of metabolism)
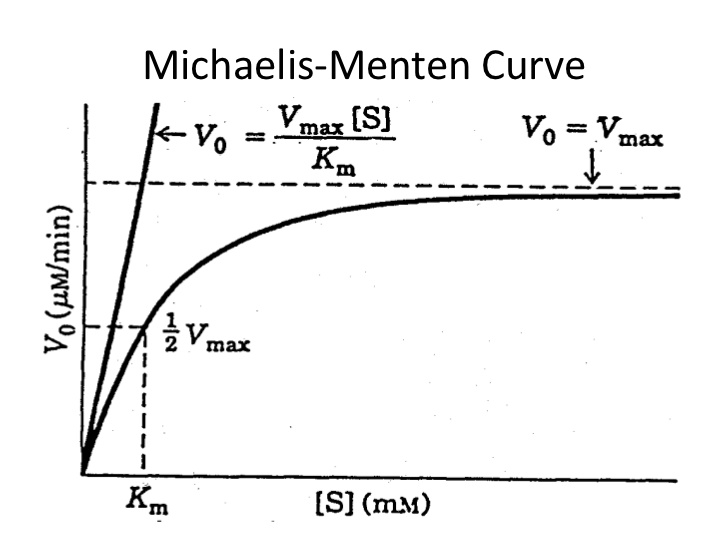
- << Km: metabolism rate mimics 1st order
- >Km: mixed order
- >> Km: zero order
- Increase in dose leads to disproportional increase in drug concentration at steady state, for this reason phenytoin dose increase should be made in small increments.
Narrow therapeutic window drugs
These drugs must stay within the safe range
|
Drug
|
Normal range
|
|
Carbamazepine
|
6-12 mcg/ml
|
|
Levothyroxine
|
FT4 0.8-1.7 mcg/dL, TSH 0.3 - 3 uIU/mL
|
|
Li
|
0.6 - 1.2 mEq/L (can be 1.5 for acute symptoms)
|
|
Phenytoin
|
10 - 20 mcg/ml
|
|
Theophylline
|
5 - 15 mcg/ml
|
|
Digoxin
|
0.8 - 2 ng/ml
|
|
Procainamide
|
4 - 12 mcg/ml
|
|
Valproic acid
|
50 - 100 mcg/ml
|
|
Warfarin
|
INR 2-3
|
Pharmacogenomics
- HLA-B testing: avoid the drug if test positive, increase the risk of hypersensitivity; Drugs: abacavir, carbamazepine/oxcarbazepam/phenytoin, allopurinol (SJS) etc.
- HER2 negative: HER2 inhibitors won’t work on patients; drugs: trastuzumab etc.
- CYP variability:
- Clopidogrel: 2C19, *2*3 are poor enzyme metabolizers, drug won’t work (increased risk of CV events); *1 is fully functional.
- Warfarin: 2C9 and VKORC, increased risk of bleeding.
- Codeine: CYP 2D6, poor metabolizer the drug won’t work.
Chemical structure
- Drugs with a sulfonamide group need to be avoided in patients with sulfa allergy.
- If the patient has an allergic reaction to procaine, other ester-type drugs should be avoided like benzocaine.
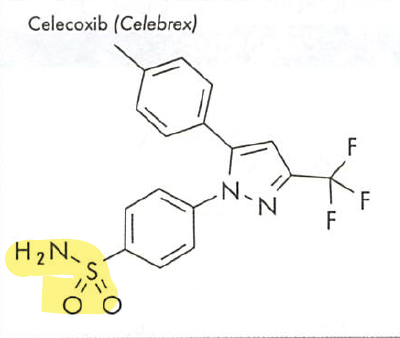
Formulations
- Sweetener examples: aspartame, saccharin, sorbitol, etc.), sorbitol produces gas, cramping bloating, especially in IBS patients.
- Lactose: commonly used in an excipient, may have tolerance issues in patients that are lactose-intolerance.
- Common patches site; chest, back, upper arm, flanks (side of the body on the abdomen level)
Phenylketonurics (PKU)
- Phenylalanine is precursor of tyrosines: NE, DA, epinephrine.
- PKU: A genetic disorder, inability to metabolize phenylalanine because of a lack of the enzyme phenylalanine hydroxylase.
- Presentation of this disease state is mental retardation, notice the above neurotransmitters – NE &DA, play huge roles in mental disorders.
- Individuals with this disorder must regulate their intake of phenylalanine.
Quiz
A patient was taking phenytoin 100mg TID and the level was found to be 8.5 mcg/L (normal range: 10-20 mcg/mL). The prescriber increased his dose to 200mg TID, the patient began to show slurred speech, fatigue, nystagmus. The repeated level was found to be 25 mcg/mL. which of the following statement is true?
- Phenytoin half life is reduced at higher doses
- Volume of distribution increases at higher doses
- Bioavailability decreases at higher doses
- Drug metabolism became saturated at higher doses
- Patient’s serum albumin level might have increased.
Answer
- D. when the dose was doubled, the metabolism became saturated and the steady state level increase dramatically.
Was this page helpful?
Back to top »
