Last updated on: Jan 11th, 2020
Background
- Immunocompromised states: diseases destroy immune response (primarily HIV patients with CD4+ T lymphocyte count<200), use of systemic steroid >14 days (>20mg/day), asplenia (no functioning spleen), use of immunosuppressants in autoimmune disorders (check chapter on autoimmune), chemotherapy.
- Opportunistic infection (OI): immunocompromised patients are unable to respond to bacteria, fungi, virus, protozoa, while healthy individuals normally are not predisposed to those risks.
- Severe neutropenia (ANC <500) is a risk factors for developing infection, without neutrophil, patients are more susceptible at gram- pathogens, virus, and fungus. Prophylaxis used in antibiotics (FQ, or 3rd generation cephalosporin), antifungal (fluconazole, or echinocandin). For medium to high risk patients, consider HSV and VZV coverage: acyclovir, valacyclovir, famciclovir; in higher risk patients, consider add mold coverage: vori/posaconazole, or amphotericin.
- HIV: an RNA virus that can insert itself into human genome (which is composed of DNA). So how can HIV survive and reproduce in host cell? In order for the RNA to be incorporated into human DNA genome, RNA has to be converted to DNA, the enzyme necessary is reverse transcriptase (normally the transcriptase converts DNA to RNA). Thus, you can already infer that most HIV drugs would target this transcription process, and block the reverse transcriptase (NRTI, NNRTI).
Screening and diagnosis of HIV
- Acute HIV symptoms are usually followed by non-specific flu like symptoms, anti-HIV antibodies (HIV ab) are undetected at the time, but the HIV RNA and p24 antigen can be detected, the Ab test usually becomes positive after 4-8 weeks of contracting the disease.
- Recommended initial screening is a combination of HIV ab and antigen immunoassay test, which detects both p24 antigen and antibodies.
- Once the initial test is positive, perform a confirmatory test to determine if the person is HIV1 or HIV2.
- OTC testing can be oral swab or fingerstick blood test, these are Ab screening test, if test sooner than 3 months of possible exposure, can lead to a false negative result.
AIDS include all HIV-infected individuals who have:
- a CD4 count < 200 cells/mm3, or
- a CD4 total lymphocyte <14% regardless of symptoms, or
- an AIDS-related opportunistic infection or condition
AIDS progression Signs and Symptoms
- Swollen lymph nodes (indicating generalized lymphadenopathy), fever (from multiple infections), constitutional symptoms (such as night sweats and fatigue), diarrhea (from infection or medications), weight loss, difficulty breathing or cough (suggesting PCP or TB), vision changes (from cytomegalovirus disease), Kaposi sarcoma (purplish skin papules are an early sign), or localized and recurrent Candidainfections, especially in women.
CD4+ counts assess the severity of disease:
- < 350 cells/mm3: patients can develop constitutional symptoms (e.g: night sweats, fatigue), herpes infections, oral candidiasis, and opportunistic infections.
- < 200 cells/mm3: at higher risk of developing Pneumocystis jiroveci(previously carinii) pneumonia (PCP) and candida (thrush). Thrush infection can resolve quickly with fluconazole, and does not require prophylactic treatment
- <100 cells/mm3: at risk for Toxoplasma gondii(causative agent of toxoplasmosis).
- < 50 cells/mm3: at higher risk for mycobacterium aviumcomplex and cytomegalovirus disease: 1st line is valganciclovir (Valcyte).
Prophylaxis of opportunistic infection
|
Pneumocystis jiroveci pneumonia (PCP)
|
CD4 <200
|
SMX /TMP DS or SS daily or dapsone
|
|
Toxoplasma gondii
|
CD4 < 100
|
SMX /TMP DS daily or dapsone + leucovorin + pyrimethamine
|
|
Mycobacterium avium complex (MAC)
|
CD4 < 50
|
Azithromycin 1200mg weekly (or 600mg BIW) or clarithromycin BID
|
Antiretroviral therapy (ART)
- Art reduces mortality and morbidity but does not cure HIV.
- The goal is to restore immune system, suppress RNA viral load to undetectable levels, ↓ mortality and morbidity, prolong survival and prevent OIs and transmission.
- Initiated in CD4 < 500 or a history of AIDS, pregnancy, HIV-associated nephropathy, hepatitis B co-infection.
- Drug resistance testing is recommended in the selection of active drugs, changing ART, and RNA level > 1000 copies/ml.
- Counseling point: Everyone misses doses occasionally; patients should achieve an adherence rate of 95% or higher for ART regimen to be effective long-term.
- One of the complications from ART is immune reconstitution inflammatory syndrome (IRIS): paradoxical worsening of an existing opportunistic infection or malignancy. Since ART improves immune, an inflammatory reaction may occur at previous infection.
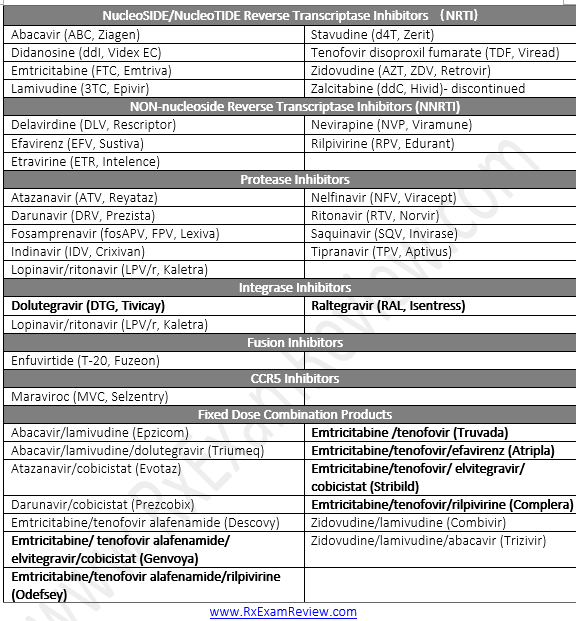
Initial regimens for HIV
- INSTI based + 2 NRTIs : better tolerability and viral suppression than PI or NNRTI based.
- dolutegravir + abacavir/lamivudine (Epzicom) = Triumeq
- dolutegravir (Tivicay) + tenofovir/emtricitabine (Truvada or Descovy)
- elvitegravir/cobicistat/tenofovir/emtricitabine (Stribild)
- raltegravir + tenofovir/emtricitabine (Truvada)
- Eivitegravir + cobicistat/emlricilabine/tenofovir disoproxil fumarate = Stribiid
- Elvitegrovlr + cobicistat/emtricitobine/tenofovir alofenamide = Genvoya
- Or 1 ritonavir-boosted PI-based regimen
- darunavir + ritonavir + tenofovir/emtricitabine (Truvada)
Initial regimen for ART naïve pregnant women
- a boosted PI (atazanavir/ritonavir Or darunavir/ritonavir) or INSTI (Raltegravir)
- + 2NRTI (abacavir/lamivudine Or Tenofovir disoproxil fumarate/emtricitabine (or lamivudine, they are interchangeable)
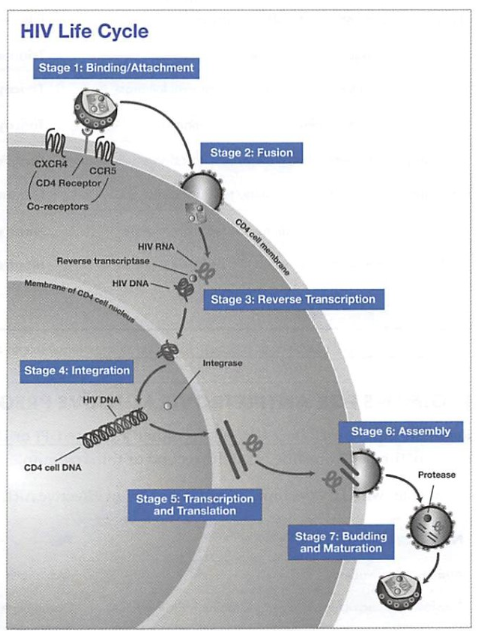
Nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs)
- Inhibits HIV reverse transcriptase, an enzyme responsible for viral replication early in the viral life cycle (# 3 on HIV lifecycle diagram).
Non-nucleoside reverse transcriptase inhibitors (NNRTIs)
- Also inhibits reverse transcriptase (#3 on HIV lifecycle diagram).
Protease inhibitors (PIs)
- Inhibitors HIV-1 protease, which is essential for viral replication in the late virus life cycle (#7 on HIV lifecycle diagram), produces immature virions.
Integrase strand transfer inhibitors (INSTIs)
- Blocks the integrase enzyme needed for viral DNA to enter the host nucleus (#4 on HIV lifecycle diagram).
Fusion inhibitors (FIs)
- Blocks fusion of virus with the CD4 cell to inhibit cell entry (#2 on HIV life cycle diagram).
CCR5 antagonists
- Blocks the CCR5 co-receptor on the cell surface which is required for HIV entry (#1 on HIV life cycle diagram).
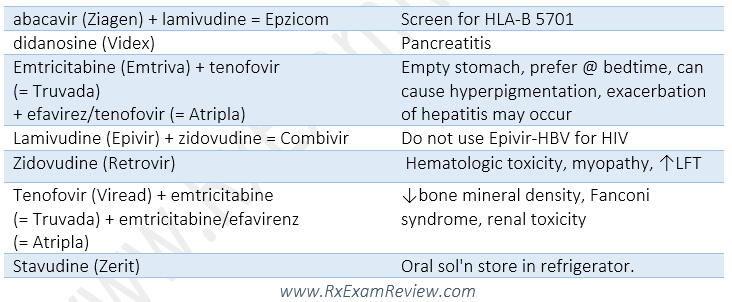
NRTI (nucleoside reverse transcriptase inhibitors)
- BBW: lactic acidosis, hepatic steatosis.
- SE: peripheral neuropathy, pancreatitis, lipoatrophy, myopathy, anemia, renal dysfunction (Elimination through kidney), liver impairment.
- Abacavir: do not use in HLA-B*5701 positive (boxed warning for fatal hypersensitivity reaction).
- emtricitabine and lamivudine are interchangeable, do not use them together.
- DI: do not undergo CYP metabolic pathway, therefore less Dis compare to PIs and NNRTIs.
Non-nucleoside reverse transcriptase inhibitors (NNRTI)
- Drugs: delavirdine (Rescriptor), efavirenz (Sustiva), etravirine (Intelence), nevirapine (Viramune), Rilpivirine (RPV, Edurant) (all has “vir” in the name)
- SE and warnings: severe skin reaction (SJS), ↑ LFTs, psychological and CNS disturbance (efavirenz, take at HS to avoid SE), QT prolongation (Rilpivirine).
- Take efavirenz on empty stomach once a day at bedtime. Efavirenz should not be used during 1st trimester, or women trying to conceive.
- With food: etravirine, rilpivirine. Rilpivirine needs an acidic environment for better absorption, contraindicated with use of PPIs.
- Nevirapine: do not use in moderate-severe hepatic impairment, women CD4>250 or men > 400.
- DI: they are all CYP substrate, or inducers (etravirine, Nevirapineg: birth control pill concentration may be reduced by inducer), or both inducer and inhibitor (efavirenz).
Protease inhibitors (PI)
- Drugs: Atazanavir, ritonavir, indinavir etc. (all end with “navir”).
- SE: GI upset (all ARTs are associated with GI toxicity with PI being the most problematic), metabolic abnormalities (hyperlipidemia, hyperglycemia, insulin insensitivity, fat redistribution/lipodystrophy (buffalo hump, increased abdominal girth, breast engorgement, fat atrophy)), ↑CV risk.
- Most PIs are taken with food for better absorption, except indinavir (Crixivan)
- Boosting agents (ritonavir and cobicistat), are only used for its potent 3A4 inhibition to increase level and efficacy of other PIs. Ritonavir is a PI, while cobicistat is not.
- Sulfa allergy caution.
- No renal dose adjustment needed for PIs.
- Atazanavir, ritonavir: need to be absorbed in an acidic environment, do not use in >20mg concurrent omeprazole.
- DI: Many drug interactions, many are 3A4 inhibitors. Be aware that a PI can be both an inhibitor and inducer, for example, ritonavir is a strong inhibitor (can raise levels of statins, PDE5 inhibitors) and is a weak inducer (can ↓ INR in patients taking warfarin due to 2C9 induction and ↓ hormonal contraceptive and methadone levels).
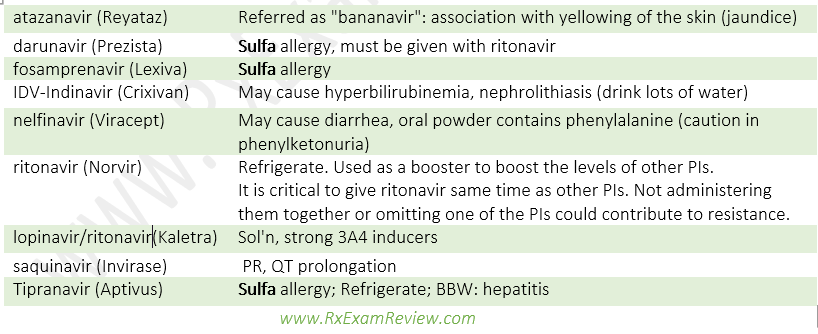
Integrase strand transfer inhibitors (INSTIs)
- Drugs: Raltegravir (Isentress, very little DDI), dolutegravir (Tivicay), elvitegravir (Vitekta), all end with “-tegravir”.
- MOA: Inhibits integrase enzyme needed for HIV DNA integration into host genome.
- SE: ↑SCr, proteinuria, ↑CPK, myopathy, rhabdomyolysis.
- DI: separate with cations (anti-acids, MVI, iron)
Fusion inhibitors
- Drug: enfuvirtide (Fuzeon)
- MOA: Inhibits the fusion process by blocking conformational change in gp41 of CD4 cells.
- Comes as self-administered injection. reconstituted sol’n must be refrigerated, injection site reactions.
CCR5 antagonist
- Drug: Maraviroc (Selzentry)
- MOA: Binds to CCR5 receptor on CD4 cell, prevent conformational change required for cell entry.
- BBW: hepatotoxicity, severe renal impairment (CrCl < 30ml/min), concurrent use of 3A4 inhibitors/inducers.
- DI: P-pg and CYP substrate.
CD4 directed post attachment HIV-1 inhibitor
- Drug: Ibalizumab-uiyk (Trogarzo) inj.
- MOA: a new class of monoclonal antibody for HIV, blocks HIV enter host cells without causing immunosuppression.
- It provides an option for patients who are multidrug-resistant to HIV.
Pre-Exposure Prophylaxis (PrEP)
- emtricitabine/tenofovir disoproxil fumarate (Truvada): FDA approved to prevent HIV infection in HIV-negative adults. 1 tab PO daily.
- Don’t suggest Emtricitabine/tenofovir (Descovy) instead of Truvada until we have efficacy data.
- Some pharmacists are prescribing PrEP using collaborative practice agreements.
- Avoid PrEP in HIV-positive patients as this could lead to resistance, advise screening for HIV before starting PrEP.
- Recommend for homosexual and heterosexual individuals at high risk for sexual exposure and IV drug users. Can take up to 3 weeks for protection from Truvada to kick in.
Post-exposure prophylaxis (PEP)
- Post-exposure includes occupational and non-occupational exposure (nPEP), the use of ART is largely the same.
- INSTI based: Raltegravir or dolutegravir + Truvada, offered as soon as possible (2 to 4 hours) after the exposure and started no later than 72 hours following the exposure.
Quiz
- A patient comes to the pharmacy with a new prescription for emtricitabine. Choose the correct drug class for emtricitabine:
- Nucleoside Reverse Transcriptase Inhibitor
- Non-Nucleoside Reverse Transcriptase Inhibitor
- CCR5 Receptor Antagonist
- Protease Inhibitor
- Integrase Inhibitor
- A patient gave the pharmacist a prescription for Prezista. Which of the following is an appropriate generic substitution for Prezista?
- Tipranavir
- Darunavir
- Maraviroc
- Emtricitabine
- Fosamprenavir
- A patient gave the pharmacist a prescription for Viread 300 mg po daily. Which of the following is an appropriate generic substitution for Viread?
- Tenofovir
- Atazanavir
- Emtricitabine
- Emtricitabine and tenofovir
- Nevirapine
- A pharmacist receives a prescription for Combivir. What medications are in this product?
- Emtricitabine + tenofovir
- Lopinavir + ritonavir
- Emtricitabine + tenofovir + rilpivirine
- Zidovudine + lamivudine
- Zidovudine + abacavir
- A physician is considering starting abacavir on a patient diagnosed with HIV. Which of the following statements regarding abacavir is correct? (Select ALL that apply.)
- This medication can cause a severe hypersensitivity reaction.
- Patients must be screened for the HLA-B 1502 allele. If positive, the medication should not be given.
- When combined with lamivudine, the brand name is Truvada.
- Abacavir is a protease inhibitor.
- The brand name is Ziagen.
- Which of the following is an acceptable antiviral regimen for a treatment-naïve patient with HIV?
- Efavirenz + nevirapine + emtricitabine
- Abacavir + didanosine + zidovudine
- Darunavir + ritonavir + atazanavir
- Raltegravir + Tenofovir + emtricitabine
- Dolutegravir + tipranavir + stavudine
- Which of the following should not be co-administered with ledipasvir/sofosbuvir due to the risk of fatal cardiac arrest?
- Tetracycline
- Simvastatin
- Amiodarone
- Ciprofloxacin
- Which of the following antibiotics is the first-line treatment for Pneumocystis jiroveci pneumonia?
A.Acyclovir
B.Azithromycin
C.Ciprofloxacin
D.Fluconazole
E. Bactrim
- Which of the following should not be coadministered with ledipasvir/sofosbuvir due to the risk of fatal cardiac arrest?
A.Doxycycline
B.Simvastatin
C.Amiodarone
Ciprofloxacin
- Which of the following is a black box warning for use of peginterferon alpha-2b injection? (Select ALL that apply.)
A.Fatal thrombocytopenia
B.Fatal neuropsychiatric disorders
C.Fatal auto-immune disorders
D.Fatal infectious disorders
E.Fatal ischemic disorders
Answers
- A. Emtricitabine (Emtriva) is a nucleoside reverse transcriptase inhibitor.
- B. Tipranavir (Aptivus), Maraviroc (Selzentry), Emtricitabine (Emtriva), Fosamprenavir (Lexiva)
- A. Atazanavir (Reyataz), Emtricitabine (Emtriva), Emtricitabine and tenofovir (Truvada), Nevirapine (Viramune).
- D. Combivir contains zidovudine (Retrovir) + lamivudine (Epivir); emtricitabine (Emtriva) + tenofovir (Viread) = Truvada; lopinavir + ritonavir (Norvir) = Kaletra, rilpivirine (Edurant) + Truvada = Complera - take with food, zidovudine (Retrovir) + abacavir (Ziagen) + lamivudine (Epivir) = Trizivir
- A, E. Abacavir (Ziagen) has a boxed warning regarding the risk of serious, and possibly fatal, hypersensitivity reaction. Every patient should be screened for the HLA-B*5701 allele. If the test is positive, the patient should not receive the drug. Abacavir + lamivudine = Epzicom. Abacavir is a NRTI.
- D. Tenofovir + emtricitabine + raltegravir is an acceptable approach for management of a treatment-naïve patient with HIV, because it contains 2 NRTIs (tenofovir and emtricitabine) and an integrase inhibitor (raltegravir). Other acceptable antiviral regimens include those that contain a protease inhibitor (PI) (boosted with ritonavir or cobicistat) + 2 NRTIs. Choice B contains 3 NRTIs (abacavir + didanosine + zidovudine). Choice A contains 2 NNRTIs (efavirenz and nevirapine) + 1 NRTI (emtricitabine). Choice C contains 3 PIs (darunavir + ritonavir + atazanavir). Choice E contains an integrase inhibitor (dolutegravir), a PI (tipranavir), and a NRTI (stavudine). They are not acceptable for treatment-naïve patient.
- C. Amiodarone can cause bradycardia, and fatal cardiac arrest can occur when ledipasvir/sofosbuvir (Harvoni) was co-administered. Harvoni is indicated for adults with chronic hepatitis C virus (HCV). If coadministration cannot be avoided, patients should have inpatient cardiac monitoring.
- E. Trimethoprim/sulfamethoxazole is the drug of choice for the treatment of Pneumocystis jiroveci pneumonia, provided that the patient does not have a sulfa allergy. Alternative medications: dapsone, pentamidine, or atovaquone, can be used for the treatment of this opportunistic infection in patients with a sulfa allergy.
- C. Amiodarone can cause symptomatic bradycardia (requiring pacemaker intervention), and fatal cardiac arrest has occurred when ledipasvir/sofosbuvir was coadministered. Bradycardia has occurred within hours to days following coadministration, and those taking beta-blockers may be at increased risk. Doxycycline, simvastatin, and levofloxacin have no known drug interactions with ledipasvir/sofosbuvir.
- BCDE. alpha-2b injection has boxed warning for neuropsychiatric, autoimmune, infectious, and ischemic events. Peg therapy should be withdrawn in patients with persistently severe or worsening signs or symptoms of these conditions. Thrombocytopenia is a rare side effect, not a boxed warning for use.
Was this page helpful?
Back to top »